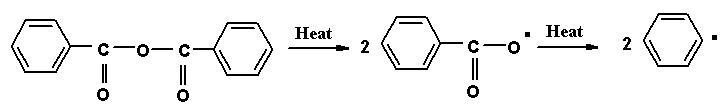
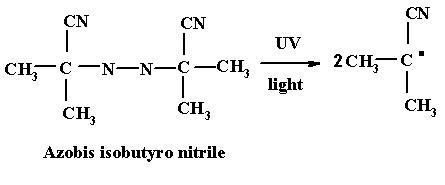
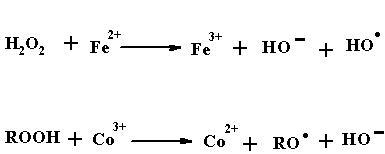Initiator
Initiators are thermally unstable compounds and decompose into products called free radicals. If R-R is an initiator and the pair of electrons forming the bond between the two R’s can be represented by dots, the initiators can be written as R:R.
When energy is supplied to this compound, in the form of heat, the molecule split into two symmetrical components, each component carries with it one of the electrons from the electron pair. This type of decomposition is called hemolytic decomposition. The two fragments, each carrying on unpaired electron with it are called free radicals i.e. R..
The decomposition of the initiator to form free radicals can be induced by heat energy, light energy or catalyst.
Decomposition by Heat energy:
Decomposition by Light Energy:
Decomposition by Catalyst: